Molar Mass Of Helium
The Universal and Individual Gas Constants in fluid mechanics and thermodynamics. Individual gas constant is given for the most common gases.
- 5 Common Uses Of Helium
- Molar Mass Of Helium Gas
- Molar Mass Of Helium In Kg
- Molar Mass Of Helium Molecule
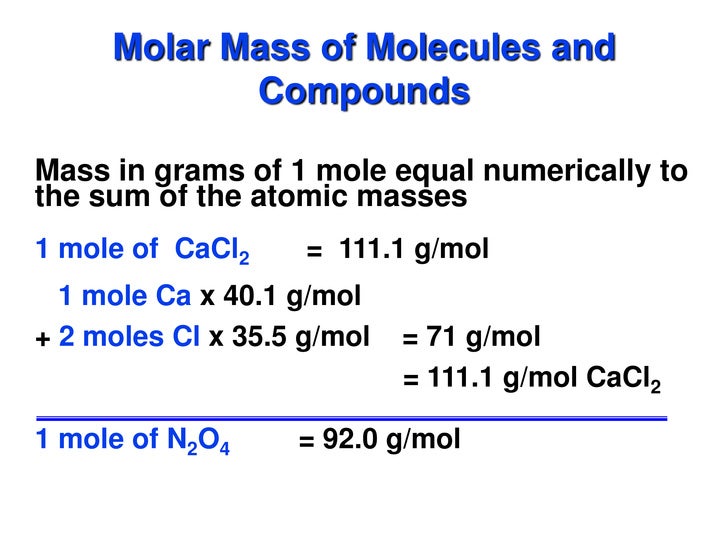
The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas constants - R - for some commonly used 'ideal gases', are in the table below (approximate values at 68 o F (20 o C) and 14.7 psia (1 atm)). Definitions of molecular mass, molecular weight, molar mass and molar weight. Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass (molar weight) is the mass of one mole of a substance and is.
The Universal and Individual Gas Constants are known from the Ideal Gas Law.
The Individual Gas Constant - R
The Individual Gas Constant depends on the particular gas and is related to the molecular weight of the gas. The value is independent of temperature. The induvidual gas constant, R, for a gas can be calculated from the universal gas constant, Ru (given in several units below), and the gas molecular weight, Mgas:
R = Ru/Mgas [1]
In the imperial system the most common units for the individual gas constant are ft lb/slug oR. In the SI system the most common units are J/kg K.
Unit conversion: 1 J/kg K = 5.97994 ft lb/slug °R, and 1 ft lb/slug °R = 0.167226 J/kg K.
The Individual Gas Constant for gases:
For full table - rotate the screen!
| Gas | Molecular Weight | Individual Gas Constant - R | |||||||
| Name | Formula | [g/mol], [kg/kmol] | [J/kg K] | [kJ/kg K] | [Wh/(kg K)] | [kcal/(kg K)], [Btu(IT)/lb °F] | [kcal/(lb °F)] | [ft lbf/lb °R] | [ft lbf/slug °R] |
| Acetylene | C2H2 | 26.038 | 319.32 | 0.3193 | 0.08870 | 0.07627 | 0.0623 | 59.350 | 1910 |
| Air | A mixture | 28.9647 | 287.05 | 0.2871 | 0.07974 | 0.06856 | 0.0560 | 53.353 | 1717 |
| Ammonia | NH3 | 17.031 | 488.21 | 0.4882 | 0.13561 | 0.11661 | 0.0952 | 90.740 | 2919 |
| Argon | Ar | 39.948 | 208.13 | 0.2081 | 0.05781 | 0.04971 | 0.0406 | 38.684 | 1245 |
| Butane | C4H10 | 58.122 | 143.05 | 0.1431 | 0.03974 | 0.03417 | 0.0279 | 26.588 | 855 |
| Butene | C4H8 | 56.106 | 148.19 | 0.1482 | 0.04116 | 0.03539 | 0.0289 | 27.543 | 886 |
| Carbon Dioxide | CO2 | 44.010 | 188.92 | 0.1889 | 0.05248 | 0.04512 | 0.0368 | 35.114 | 1130 |
| Carbon Monoxide | CO | 28.010 | 296.84 | 0.2968 | 0.08246 | 0.07090 | 0.0579 | 55.171 | 1775 |
| Carbonic acid | H2CO3 | 62.025 | 134.05 | 0.1341 | 0.03724 | 0.03202 | 0.0261 | 24.915 | 802 |
| Chlorine | Cl2 | 70.906 | 117.26 | 0.1173 | 0.03257 | 0.02801 | 0.0229 | 21.794 | 701 |
| Chloromethane | CH3Cl | 50.488 | 164.68 | 0.1647 | 0.04575 | 0.03933 | 0.0321 | 30.608 | 985 |
| Dichlorofluorumethane | CHCl2F | 102.923 | 80.78 | 0.0808 | 0.02244 | 0.01929 | 0.0158 | 15.015 | 483 |
| Ethane | C2H6 | 30.069 | 276.51 | 0.2765 | 0.07681 | 0.06604 | 0.0539 | 51.393 | 1654 |
| Ethene | C2H4 | 28.053 | 296.38 | 0.2964 | 0.08233 | 0.07079 | 0.0578 | 55.086 | 1772 |
| Fluorine | F2 | 37.997 | 218.82 | 0.2188 | 0.06078 | 0.05226 | 0.0427 | 40.670 | 1309 |
| Helium | He | 4.003 | 2077.1 | 2.0771 | 0.57696 | 0.49610 | 0.4050 | 386.047 | 12421 |
| Hydrogen | H2 | 2.016 | 4124.2 | 4.1242 | 1.14563 | 0.98506 | 0.8043 | 766.541 | 24663 |
| Hydrogen bromide | HBr | 80.912 | 102.76 | 0.1028 | 0.02854 | 0.02454 | 0.0200 | 19.099 | 614 |
| Hydrogen chloride | HCl | 36.461 | 228.04 | 0.2280 | 0.06334 | 0.05447 | 0.0445 | 42.384 | 1364 |
| Hydrogen sulfide | H2S | 34.081 | 243.96 | 0.2440 | 0.06777 | 0.05827 | 0.0476 | 45.344 | 1459 |
| Krypton | Kr | 83.798 | 99.22 | 0.0992 | 0.02756 | 0.02370 | 0.0193 | 18.441 | 593 |
| Methane (natural gas) | CH4 | 16.042 | 518.28 | 0.5183 | 0.14397 | 0.12379 | 0.1011 | 96.329 | 3099 |
| Neon | Ne | 20.180 | 412.02 | 0.4120 | 0.11445 | 0.09841 | 0.0803 | 76.579 | 2464 |
| Nitrogen | N2 | 28.013 | 296.80 | 0.2968 | 0.08245 | 0.07089 | 0.0579 | 55.165 | 1775 |
| Nitrogen dioxide | NO2 | 46.006 | 180.73 | 0.1807 | 0.05020 | 0.04317 | 0.0352 | 33.590 | 1081 |
| Nitrogen trifluoride | NF3 | 71.002 | 117.10 | 0.1171 | 0.03253 | 0.02797 | 0.0228 | 21.765 | 700 |
| Nitrous oxide | N2O | 44.012 | 188.91 | 0.1889 | 0.05248 | 0.04512 | 0.0368 | 35.112 | 1130 |
| Oxygen | O2 | 31.999 | 259.84 | 0.2598 | 0.07218 | 0.06206 | 0.0507 | 48.294 | 1554 |
| Propane | C3H8 | 44.096 | 188.56 | 0.1886 | 0.05238 | 0.04504 | 0.0368 | 35.045 | 1128 |
| Propene | C3H6 | 42.080 | 197.59 | 0.1976 | 0.05489 | 0.04719 | 0.0385 | 36.724 | 1182 |
| Sulfur dioxide | SO2 | 64.064 | 129.78 | 0.1298 | 0.03605 | 0.03100 | 0.0253 | 24.122 | 776 |
| Sulfur hexafluoride | SF6 | 146.055 | 56.93 | 0.0569 | 0.01581 | 0.01360 | 0.0111 | 10.581 | 340 |
| Sulfur trioxide | SO3 | 80.063 | 103.85 | 0.1038 | 0.02885 | 0.02480 | 0.0203 | 19.302 | 621 |
| Water vapor | H2O | 18.015 | 461.52 | 0.4615 | 0.12820 | 0.11023 | 0.0900 | 85.780 | 2760 |
| Xenon | Xe | 131.293 | 63.33 | 0.0633 | 0.01759 | 0.01513 | 0.0123 | 11.770 | 379 |
The Universal Gas Constant - Ru
The Universal Gas Constant - Ru - appears in the ideal gas law and can be expressed as the product between the Individual Gas Constant - R - for the particular gas - and the Molecular Weight - Mgas - for the gas, and is the same for all ideal or perfect gases:
5 Common Uses Of Helium
Ru = Mgas R [2]
The Universal Constant defined in Terms of the Boltzmann's Constant
The universal gas constant can be defined in terms of Boltzmann's constant k as:
Ru = k NA [3]
where
k = Boltzmann's constant = 1.381 x 10-23 [J/K]
NA = Avogadro Number = 6.022 x 1023 [1/mol]
Molar Mass Of Helium Gas
The Molecular weight of a Gas Mixture
The average molecular weight of a gas mixture is equal to the sum of the mole fractions of each gas multiplied by the molecular weight of that particular gas:
Mmixture = Σxi*Mi = (x1*M1 + ......+ xn*Mn) [4]
where
xi = mole fractions of each gas
Mi = the molar mass of each gas
The Universal Gas Constant - Ru - in alternative Units
- atm.cm3/(mol.K) : 82.057338
- atm.ft3/(lbmol.K) : 1.31443
- atm.ft3/(lbmol.oR) : 0.73024
- atm.l/(mol.K) : 0.082057338
- bar.cm3/(mol.K) : 83.144598
- bar.l/(mol.K) : 0.083144598
- Btu/(lbmol.oR) : 1.9872036
- cal/(mol.K) : 1.9859
- erg/(mol.K) : 83144598
- hp.h/(lbmol.oR) : 0.0007805
- inHg.ft3/(lbmol.oR) : 21.85
- J/(mol.K) : 8.3144598
- kJ/(kmol.K) : 8.3144598
- J/(kmol.K) : 8314.472
- (kgf/cm2).l/(mol.K) : 0.084784
- kPa.cm3/(mol.K) : 8314.4598
- kWh/(lbmol.oR) : 0.000582
- lbf.ft/(lbmol.oR) : 1545.349
- mmHg.ft3/(lbmol.K) : 999
- mmHg.ft3/(lbmol.oR) : 555
- mmHg.l/(mol.K) : 62.363577
- Pa.m3/(mol.K) : 8.3144598
- psf.ft3/(lbmol.oR) : 1545.3465
- psi.ft3/(lbmol.oR) : 10.73
- Torr.cm3/(mol.K) : 62364
See also:
- More material properties
- The Ideal Gas Law - Gases are highly compressible with changes in density directly related to changes in temperature and pressure.
- A Mixture of Gases - Properties of mixtures of gases.
- More about temperature
Related Topics
- Fluid Mechanics - The study of fluids - liquids and gases. Involves velocity, pressure, density and temperature as functions of space and time
- Gases and Compressed Air - Air, LNG, LPG and other common gas properties, pipeline capacities, sizing of relief valves
- Air Psychrometrics - The study of moist and humid air - psychrometric charts, Mollier diagrams, air-condition temperatures and absolute and relative humidity and moisture content
- Material Properties - Material properties for gases, fluids and solids - densities, specific heats, viscosities and more
Related Documents
- Acetone - Thermophysical Properties - Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included.
- Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight (or molar mass) can be calculated by adding the weight of each component
- Air - Thermophysical Properties - Thermal properties of air - density, viscosity, critical temperature and pressure, triple point, enthalpi and entropi, thermal conductivity and diffusicity, and more
- Benzene - Thermophysical properties - Chemical, physical and thermal properties of benzene, also called benzol. Phase diagram included.
- Dry Air Properties - Dry air properties at temperatures ranging 175 - 1900 K - specific heat, ratio of specific heats, dynamic viscosity, thermal conductivity, Prandtl number, density and kinematic viscosity
- Ethylene - Thermophysical Properties - Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas. Phase diagram included.
- Gas Mixture Properties - Special care must be taken for gas mixtures when using the ideal gas law, calculating the mass, the individual gas constant or the density
- Gases - Dynamic Viscosity - Absolute viscosities of gases
- Gases - Molar Specific Heat - Molar specific heats of gases at constant volume
- Humid Air and the Ideal Gas Law - Pressure, temperature and volume for an ideal or perfect gas like air with water vapor - or moist air
- Ideal Gas Law - The relations between volume, pressure, temperature and quantity of a gas, including definition of density of a gas
- Mole Fraction of Water Vapor in Moist Air - Mole fraction of water vapor is the ratio of water molecules - to air and water molecules
- Mollier Diagram for Water-Steam - Enthalpy-entropy diagram for water and steam
- Nitrogen - Enthalpy, Internal Energy and Entropy - Enthalpy, internal energy and entropy of Nitrogen as ideal gas
- Non-ideal gas - Van der Waal's Equation and Constants - Listing of van der Waals constants for more than 200 gases, used to correct for non-ideal behavior of gases caused by intermolecular forces and the volume occupied by the gas particles
- Rankine Efficiency - The efficiency of the Rankine cycle
- Ratios of Specific Heat of Gases - Ratios of specific heat for gases in constant pressure and volume processes
- Sulfur Dioxide Liquid - Thermal Properties - Density, specific heat, thermal conductivity and more
- Temperature - Introduction to temperature - including Celsius, Fahrenheit, Kelvin and Rankine definitions - an online temperature converter
- Thermodynamic Terms, Functions and Relations - Common thermodynamic terms and functions - potential energy, kinetic energy, thermal or internal energy, chemical energy, nuclear energy and more
- Total and partial pressure - Dalton's law of partial pressures - How to calculate total pressure and partial pressures for gas mixtures from Ideal Gas Law
Tag Search
- en: individual universal gas constant R air helium
Graham's Law of Effusion
Examples and Problems only
Example #1: 8.278 x 10¯4 mol of an unidentified gaseous substance effuses through a tiny hole in 86.9 s Under identical conditions, 1.740 x 10¯4 mol of argon gas takes 81.3 s to effuse.
a) What is the molar mass of the unidentified substance (in g/mol)?
b) What is the molecular formula of the substance?
c) Under identical conditions, how many moles of ethene (C2H4) gas would effuse in 91.0 s?
Example #2: It takes 354 seconds for 1.00 mL of Xe to effuse through a small hole. Under the same conditions, how long will it take for 1.00 mL of nitrogen to effuse?
Example #3: What is the rate of effusion for a gas that has a molar mass twice that of a gas that effuses at a rate of 4.2 mol/min?
Example #4: It takes 110. seconds for a sample of carbon dioxide to effuse through a porous plug and 275 seconds for the same volume of an unknown gas to effuse under the same conditions. What is the molar mass of the unknown gas (in g/mol)?
Example #5: What is the molar mass of a compound that takes 2.65 times as long to effuse through a porous plug as it did for the same amount of XeF2 at the same temperature and pressure?
Example #6: If a gas effuses 4.25 times faster than iodine gas (I2), what is its molar mass?
A. 59.7 g/mol
B. 163 g/mol
C. 123 g/mol
D. 158 g/mol
Example #7: Calculate the density of a gas at STP, if a given volume of the gas effuses through an apparatus in 6.60 min and the same volume of nitrogen, at the same temperature andpressure, effuses through this apparatus in 8.50 minutes.
Example #8: If 0.0949 moles of NH3 effuses in 881 seconds, how many seconds would it take for the same number of moles of B2H6 to effuse?
Example #9: The rate of diffusion of an unknown gas was determined to be 2.92 times greater than that of NH3. What is the approximate molar mass of the unknown gas?
Example #10: If a molecule of C2H6 diffuses a distance of 0.978 m from a point source, calculate the distance (m) that a molecule of CH4 would diffuse under the same conditions for the same period of time.
Example #11: A sample of oxygen gas (O2) effuses into a vacuum 1 times faster than an unknown gas. O2 has a molecular weight of about 32.00 g mol¯1. What is the molecular weight of this unknown gas (in g mol¯1)?
Example #12: Argon effuses from a container at a rate of 0.0260 L/s. How long will it take for 3.00 L of I2 to effuse from the same container under identical conditions?
Example #13: Calculate the density of a gas at STP, if a given volume of the gas effuses through an apparatus in 6.60 min and the same volume of oxygen at the same temperature and pressure, effuses through this apparatus in 8.50 minutes.
Bonus Example #1: The rate of effusion of an unknown gas at 480 K is 1.6 times the rate of effusion of SO2 gas at 300 K. Calculate the molecular weight of the unknown gas.
Bonus Example #2: Heavy water, D2O (molar mass = 20.0276 g mol¯1), can be separated from ordinary water, H2O (molar mass = 18.0152 g mol¯1), as a result of the difference in the relative rates of diffusion of the molecules in the gas phase. Calculate the relative rates of diffusion for H2O when compared to D2O.
Problem #1: If equal amounts of helium and argon are placed in a porous container and allowed to escape, which gas will escape faster and how much faster?
Problem #2: What is the molecular weight of a gas which diffuses 1/50 as fast as hydrogen?
Problem #3: Two porous containers are filled with hydrogen and neon respectively. Under identical conditions, 2/3 of the hydrogen escapes in 6 hours. How long will it take for half the neon to escape?
Problem #4: If the density of hydrogen is 0.090 g/L and its rate of effusion is 5.93 times that of chlorine, what is the density of chlorine?
Problem #5: How much faster does hydrogen escape through a porous container than sulfur dioxide?
Problem #6: Compare the rate of diffusion of carbon dioxide (CO2) & ozone (O3) at the same temperature.
Problem #7: 2.278 x 10¯4 mol of an unidentified gaseous substance effuses through a tiny hole in 95.70 s. Under identical conditions, 1.738 x 10¯4 mol of argon gas takes 81.60 s to effuse. What is the molar mass of the unidentified substance?
Problem #8: A compound composed of carbon, hydrogen, and chlorine diffuses through a pinhole 0.411 times as fast as neon. Select the correct molecular formula for the compound:
(a) CHCl3
(b) CH2Cl2
(c) C2H2Cl2
(d) C2H3Cl
Problem #9: Which pair of gases contains one which effuses at twice the rate of the other in the pair?
(a) He and Ne
(b) Ne and CO2
(c) He and CH4
(d) CO2 and HCl
(e) CH4 and HCl
Problem #10: If a molecule of CH4 diffuses a distance of 0.530 m from a point source, calculate the distance (in meters) that a molecule of N2 would diffuse under the same conditions for the same period of time.
Bonus Problem #1: Calculate the density of a gas at STP, if a given volume of the gas effuses through an apparatus in 6.60 min and the same volume of nitrogen at the same temperature and pressure, effuses through this apparatus in 8.50 minutes.
Bonus Problem #2: At 25.0 °C and 380.0 mmHg, the density of sulfur dioxide is 1.31 g/L. The rate of effusion of sulfur dioxide through an orifice is 4.48 mL/s. (a) What is the density of a sample of gas that effuses through an identical orifice at the rate of 6.78 mL/s under the same conditions? (b) What is the molar mass of the gas?
Problem #11: What is the rate of effusion for a gas that has a molar mass twice that of a gas that effuses at a rate of 3.62 mol/min?
Problem #12: Calculate the rate of effusion of NO2 compared to SO2 at the same temperature and pressure.
Problem #13: Assume you have a sample of hydrogen gas containing H2, HD, and D2 that you want to separate into pure components. What are the various ratios of relative rates of effusion?
Molar Mass Of Helium In Kg
Problem #14: A 3.00 L sample of helium was placed in container fitted with a porous membrane. Half of the helium effused through the membrane in 25 hours. A 3.00 L sample of oxygen was placed in an identical container. How many hours will it take for half of the oxygen to effuse though the membrane?
Problem #15: At a certain temperature, hydrogen molecules move at an average velocity of 1.84 x 103 m/s. Estimate the molar mass of a gas whose molecules have an average velocity of 311 m/s.
Problem #16: An unknown gas effuses 1.66 times more rapidly than CO2. What is the molar mass of the unknown gas.
Problem #17: A sample of hydrogen gas effuse through a porous container 8.91 times faster than an unknown gas. Estimate the molar mass of the unknown gas.
Problem #18: N2 is contaminated with a noble gas.The contaminant effuses at 1.87x N2. What is the noble gas?
Problem #19: In an effusion experiment, it was determined that nitrogen gas, N2, effused at a rate 1.812 times faster than an unknown gas. What is the molar mass of the unknown gas?
Problem #20: Why are the rates of diffusion of nitrogen gas and carbon monoxide almost identical at the same temperature?
Problem #21: In running a diffusion experiment, ammonia is found to diffuse 30.0 cm during the same amount of time hydrogen chloride moves 20.0 cm. Calculate the percentage deviation from Graham's Law.
Problem #22: A sample of Br2(g) take 10.0 min to effuse through a membrane. How long would it take the same number of moles of Ar(g) to effuse through the same membrane?
Problem #23: At a particular pressure and temperature, it takes just 8.256 min for a 4.893 L sample of Ne to effuse through a porous membrane. How long would it take for the same volume of I2 to effuse under the same conditions?
Problem #24a: How much faster does U235F6 effuse than U238F6?
Problem #24b: Calculate the ratio of effusion rates for U238F6 and U235F6. Express your answer using five significant figures and as the following ratio:
Molar Mass Of Helium Molecule
rate U238F6 / rate U235F6
Problem #25: O3 effuses 0.8165 times as fast as O2. What % of the molecules effusing first would be O2?
Bonus Problem #1: HCl and NH3 diffuse through a tube and a white disc of NH4Cl is formed. Where in the tube?
Bonus Problem #2: One way of separating oxygen isotopes is by gaseous diffusion of carbon monoxide. The gaseous diffusion process behaves like an effusion process. Calculate the relative rates of effusion of:
12C16O
12C17O
12C18O
